Mitonic® Pen (MOTS-c)
Mitonic® in injection contains MOTS-c. Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c)
is a mitochondrial-derived peptide composed of 16 amino acids. The MOTS-c effect includes:
• prevented the development of heart failure, improve heart structure and function,
thereby protecting the health of cardiovascular
• enhances insulin sensitivity throughout the body through muscles and improve the utilization
of glucose
• suppression of inflammation, a significant decrease in pro-inflammatory cytokines
and an increase in anti-inflammatory cytokines
• MOTS-c is described as a "motion simulator," meaning that its use increases physical tone
and increases motivation for physical activity
• directly, by regulating glucose sensitivity, and indirectly, by stimulating metabolism
and physical activity, it reduces body weight and body fat
• strengthens bones and prevents bone loss after illnesses
MECHANISM OF ACTION
Mitochondrial-derived
peptides are a family of peptides encoded by short open reading frames in the mitochondrial
genome, which have regulatory effects on mitochondrial functions, gene expression,
and metabolic homeostasis of the body. Mitochondrial open reading frame of the 12S
rRNA-c, MOTS-c is the active ingredient of Mitonic®, as a new member of the
mitochondrial-derived peptide family, is regarding a peptide hormone
that could reduce insulin resistance, prevent obesity, improve muscle function,
promote bone metabolism, enhance immune regulation, and postpone aging.
MOTS-c plays these physiological functions mainly through activating the
AICAR-AMPK signaling pathways by disrupting the folate-methionine cycle in cells.
Recent studies have shown that the above hormonal effect can be achieved through
MOTS-c regulating the expression of genes such as GLUT4, STAT3, and IL-10.
PHARMACODYNAMICS
Сompensation for age-related changes in muscle tissue
A gradual metabolic decline is one of the hallmarks of aging, suppressing normal
physiological functions and even leading to the loss of self-care. Aging is a key
risk factor for chronic diseases. Adaptation of cellular responses to changing
internal and external conditions is essential for the body's health. In addition
to generating large amounts of cellular energy, mitochondria are closely linked
to aging.
Studies have shown that MOTS-c can improve the expression of mitochondrial
protective genes (Kim KH, Son JM, Benayoun BA and Lee C). The aging process
can lead to a decrease in MOTS-c levels (Kim SJ, Miller B, Kumagai H, Silverstein AR,
Flores M and Yen K). In fact, MOTS-c levels in skeletal muscle and circulation
in both humans and mice decline with age. Studies have shown that MOTS-c levels
in the blood of young adults are 11% and 21% higher than those of middle-aged and
elderly people, respectively (D'Souza RF, Woodhead JST, Hedges CP, Zeng N,
Wan J and Kumagai H). Furthermore, the strong correlation between the increase
in pathological effects with age and MOTS-c levels suggests that higher MOTS-c
levels are beneficial in slowing down aging.
Preventing obesity and insulin resistance
MOTS-c was also able to prevent high-fat diet-induced
obesity and hyperinsulinemia, independent of calorie intake. Studies (Lee C, Zeng J,
Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R,
Cohen P) report that MOTS-c prevents high-fat diet-induced obesity by increasing
energy expenditure, including heat production, and improving glucose utilization
and insulin sensitivity. The reduction in fat accumulation may be due to increased
carbohydrate consumption, which reduces fatty acid synthesis, but increased fatty
acid oxidation observed in vitro may also contribute, but requires detailed study
before it can be ruled out.
Slows the loss of muscle speed-strength qualities with age
As is well known, the ratio of muscle fiber types
changes with age. In older individuals, the proportion of muscle fibers responsible
for strong and high-speed (explosive) muscle contractions decreases, while
the proportion of so-called "slow-twitch" fibers responsible for prolonged,
low-intensity work increases. Administration of exogenous MOTS-c can slow
the process of replacing fast-twitch fibers with slow-twitch ones, and consequently,
the decline in muscle speed and strength.
Improving the quality and contractile function of muscles while maintaining constant muscle mass
In the maximum weight leg press, participants with a higher concentration
of MOTS-c in their muscles showed higher results than those with a lower
level of MOTS-c.
Reducing myostatin levels and the toxic effects of obesity and high-fat diets on muscle fibers
Myostatin is a key mediator of skeletal muscle atrophy
caused by insulin resistance. Myostatin levels are elevated in mice fed a high-fat
diet and in obese individuals. MOTS-c has been shown to mimic exercise in mice fed
a high-fat diet (HFD), improving insulin sensitivity and preventing weight gain.
These results suggest that MOTS-c likely reduces myostatin levels by inhibiting
myostatin production in skeletal muscle.
Suppression of inflammatory reactions
A significant reduction in pro-inflammatory cytokines
and an increase in anti-inflammatory cytokines were observed with MOTS-c injections.
Furthermore, an increase in the content and activity of substances responsible
for the analgesic effect was recorded.
MOTS-c and cognitive function
MOTS-c enhanced the formation and consolidation
of object and location recognition memory and improved age-related memory deficits.
EFFICIENCY BASED ON RESEARCH DATA
֍ MOTS-c improves metabolic
homeostasis and reduces insulin resistance
Initial studies of MOTS-c found modest reductions in body weight, food intake,
and blood glucose levels in MOTS-c-treated mice fed a high-fat diet (HFD) [ Lee C,
Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. ]. MOTS-c treatment
enhanced cellular glucose flux in vitro and reduced glucose levels in mice fed
a normal diet. Significantly increased glucose clearance in the glucose tolerance
test and hyperinsulin-orthoglycemic clamp studies demonstrated improved systemic
insulin sensitivity [ Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J
, et al. ]. Furthermore, enhanced skeletal muscle-specific insulin sensitivity was
demonstrated by deuterated glucose injection [Lee C, Zeng J, Drew BG, Sallam T,
Martin-Montalvo A, Wan J, et al.]. Interestingly, muscles from old mice were more
insulin resistant than muscles from young mice, but MOTS-c treatment restored the
sensitivity of old mice to a level comparable to that of young mice. Although MOTS-c
treatment had no effect on body weight in mice fed a normal diet, when administered
to mice fed a high-fat diet, it significantly reduced the incidence of obesity and
basal levels of circulating IL-6 and TNF-α, which are associated with the pathogenesis
of obesity and insulin resistance. Furthermore, MOTS-c treatment prevented high-fat
diet-induced hyperinsulinemia, indicating improved glucose homeostasis [ Lee C, Zeng J,
Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. ]. Overall, MOTS-c prevented
high-fat diet-induced obesity by increasing energy expenditure, improving glucose
utilization, and insulin sensitivity.
Postmenopausal women are known to experience physiological changes, including
weight gain, changes in adipose tissue distribution, and decreased insulin secretion
and sensitivity. MOTS-c treatment prevents postmenopausal obesity and insulin
resistance [Kim SJ, Miller B, Kumagai H, Yen K, Cohen P]. MOTS-c prevents weight
gain and reduces obesity in ovariectomized mice (used to mimic menopause)
by reducing blood lipid levels and liver triacylglycerol levels, and enhances
lipolysis [Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, et al.]. Interestingly,
these experiments also showed that MOTS-c treatment significantly downregulated
adipogenesis-related genes ( Fasn, Scd1 ) and upregulated lipid oxidation-related
genes.
In type 2 diabetes (T2D) and obesity, three sphingolipid
metabolism pathways, monoacylglycerol metabolism and dicarboxylic acid metabolism,
are activated. Importantly, MOTS-c improves insulin sensitivity, enhances β-oxidation,
and prevents fat accumulation in mice by suppressing these pathways [ Kim SJ, Miller B,
Mehta HH, Xiao J, Wan J, Arpawong TE, et al. ]. This suggests that MOTS-c may improve
insulin resistance by reducing the levels of sphingolipid metabolites. In MOTS-c-treated
mice, increased ANGPTL4 levels may inhibit LPL and prevent fat accumulation in muscle,
thereby improving insulin sensitivity [ Kim SJ, Miller B, Mehta HH, Xiao J, Wan J,
Arpawong TE, et al. ]. Elevated levels of dicarboxylic acids (DCAs) are generally
considered a sign of dysregulated mitochondrial and peroxisomal β-oxidation.
Reduced plasma DCA levels in MOTS-c-treated mice indicate a higher efficiency
of normal β-oxidation. In a recent study, MOTS-c treatment significantly reduced
hyperglycemia and improved insulin sensitivity in gestational diabetic mice,
and decreased mortality in offspring [ Yin Y, Pan Y, He J, Zhong H, Wu Y, Ji C,
et al. ]. In conclusion, there are more ways in which MOTS-c may regulate metabolic
homeostasis and insulin resistance, and this is a promising direction for future
research.
֍ Studies of the anti-inflammatory
and immune effects of MOTS-c
Inflammation is an evolutionarily conserved defense
mechanism whose purpose is to maintain the stability of the body's internal environment
in the face of infection or injury. However, overproduction of proinflammatory factors
and overreactive immune responses can lead to multiorgan dysfunction and tissue damage.
As described above, MOTS-c can activate several molecules, such as AMPK, SIRT1, and
NF-κB, while simultaneously inhibiting ROS production, suggesting a potential
anti-inflammatory effect of MOTS-c, which is also consistent with a large number
of subsequent studies. For example, in methicillin-resistant Staphylococcus aureus
(MRSA) sepsis, MOTS-c significantly increased survival and reduced bacterial load
in mice while simultaneously decreasing proinflammatory cytokine levels (TNF-α,
IL-6, IL-1β) and increasing anti-inflammatory cytokine IL-10 levels [Zhai D,
Ye Z, Jiang Y, Xu C, Ruan B, Yang Y, et al.]. Similarly, in an inflammatory
injury pain model, MOTS-c significantly inhibited formalin-induced ERK, JNK,
and P38 activation, as well as c-Fos expression (recognized as an important
mediator of inflammatory pain). This suggests that MOTS-c may exert
anti-inflammatory effects by inhibiting the MAPK-c-Fos signaling pathway
and reducing inflammation-induced pain stimulation. Furthermore, MOTS-c
was found to exert the same effect in an acute lung injury model, reducing
pulmonary edema and inhibiting neutrophil infiltration into lung tissue
[Xinqiang Y, Quan C, Yuanyuan J, Hanmei X].
In addition to regulating the expression of
proinflammatory factors, MOTS-c can also exert anti-inflammatory effects
by targeting immune cells (such as T cells and macrophages). A recent
study showed that MOTS-c treatment reduced the number of islet-infiltrating
T cells and prevented islet β-cell destruction, thereby slowing the progression
of non-obese diabetes (NOD) [Kong BS, Min SH, Lee C, Cho YM]. In particular,
MOTS-c promotes the differentiation of regulatory T cells, which exhibit low
glycolytic activity and demonstrate therapeutic potential in type 1 diabetes
(T1D) and other autoimmune diseases [ Bluestone JA, Buckner JH, Fitch M, Gitelman SE,
Gupta S, Hellerstein MK, et al. ].
MOTS-c did not increase the number of macrophages in
uninfected mice, but it did enhance phagocytic and bactericidal capacity [ Zhai D
Ye Z, Jiang Y, Xu C, Ruan B, Yang Y, et al. ].
According to preclinical studies, postmenopausal
women are at increased risk of obesity, insulin resistance, osteoporosis,
cardiovascular disease, and cognitive decline. Currently, the mainstay of
treatment for postmenopausal pathologies is hormone therapy, but its risks
and benefits remain controversial [ Pinkerton JV. Hormone therapy for
postmenopausal women ]. On the contrary, the discovery of MOTS-c may provide
a promising treatment or adjunctive therapy for postmenopausal women.
Interestingly, when MOTS-c was fused with the
cell-penetrating peptide (PRR) 5 to cross the blood-brain barrier, it enhanced
the formation and consolidation of object and location recognition memories and
ameliorated memory deficits induced by Aβ1-42 or LPS [Jiang J, Chang X, Nie Y,
Shen Y, Liang X, Peng Y, et al.]. MOTS-c treatment significantly reduced the
expression of proinflammatory cytokines (including IL-6, IL-1β, and TNF-α)
in the hippocampus after LPS or Aβ1 treatment.
֍MOTS-c in exercise and aging
MOTS-c has potential roles in promoting healthy
aging, such as maintaining homeostasis of the body, improving physical function,
and alleviating aging-related pathologies. Indeed, recent studies support this
view. For example, MOTS-c significantly improved the physical function in mice
of all ages. MOTS-c regulates the expression of genes related to metabolism and
protein stabilization, skeletal muscle metabolism, and myocyte adaptation to stress.
Moreover, exercise promoted the expression of endogenous MOTS-c and increased the
level of MOTS-c in skeletal muscle and plasma (returning to initial levels after
4 h of rest) [Reynolds JC, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D,
et al]
There is growing evidence that acute and long-term
exercise can modulate endogenous MOTS-c levels. Concomitantly, treatment with
exogenous MOTS-c improves physical function. MOTS-c treatment significantly
improved physical performance (significantly longer running time, increased
endurance, increased maximum speed) in aged mice, in part by modulating skeletal
muscle function and improving "metabolic flexibility." Body motor function is
essential for healthy aging, and MOTS-c treatment of aged mice resulted in a
trend toward increased mean and maximum lifespan with a reduced hazard ratio
[Reynolds JC, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, et al.].
This suggests that MOTS-c may serve as a potential treatment for muscle atrophy.
MOTS-c enhances glycolytic flux and energy production in Duchenne muscular dystrophy
(DMD)-affected muscles, in addition to improving muscle capacity in healthy mice
[Ran N, Lin C, Leng L, Han G, Geng M, Wu Y, et al.].
DOSAGE AND ADMINISTRATION
Dosage
• The first recommended starting dosage of Mitonic® (MOTS-c) is 1 mg injected.
This dose is necessary at the beginning of treatment to minimize the negative
sensations and to identify individual reactions.
• If a single dose of 1 mg is well tolerated, use the current dosage of
1-4 mg every day or every other day.
• Perform subcutaneous injections into the abdomen and thighs. Try to avoid
intramuscular injections for more uniform distribution into the blood plasma.
•
The maximum dosage of Mitonic® (MOTS-c) is 4 mg.
Important Administration Instructions
• Administer Mitonic®, any time of day, with or without meals.
• Inject Mitonic® subcutaneously in the abdomen, thigh, or upper arm.
• Try to avoid intramuscular injections for more uniform distribution into the blood plasma.
• Rotate injection sites with each dose.
• Do not mix Mitonic® with other injectable medications.
• Give injections at least 30 minutes apart.
• Maintain a distance of at least 5 centimeters between injections.
CONTRAINDICATIONS AND ADVERSE REACTIONS
Minimal side effects and reactions were observed
during treatment with MOTS-c. The most common side effect was dizziness immediately
after the injection. The severity of this reaction decreased over time with regular use.
Redness and itching may occur at the injection site.
Skin hypersensitivity, both at the injection site
and generally, is possible. This reaction persisted for 2-3 weeks after discontinuing
MOTS-c.
Mitonic® is contraindicated in patients with:
• In case of known sensitivity to MOTS-c and other components of Mitonic®.
• History of rhabdomyosarcoma or other forms of muscle tissue degeneration in patients.
• With acute pancreatic reactions until stabilization of enzyme levels in the blood is achieved.
INGREDIENTS
Active ingredient: MOTS-c
Inactive ingredients: sodium chloride, sodium acetate, methionine, phenol.






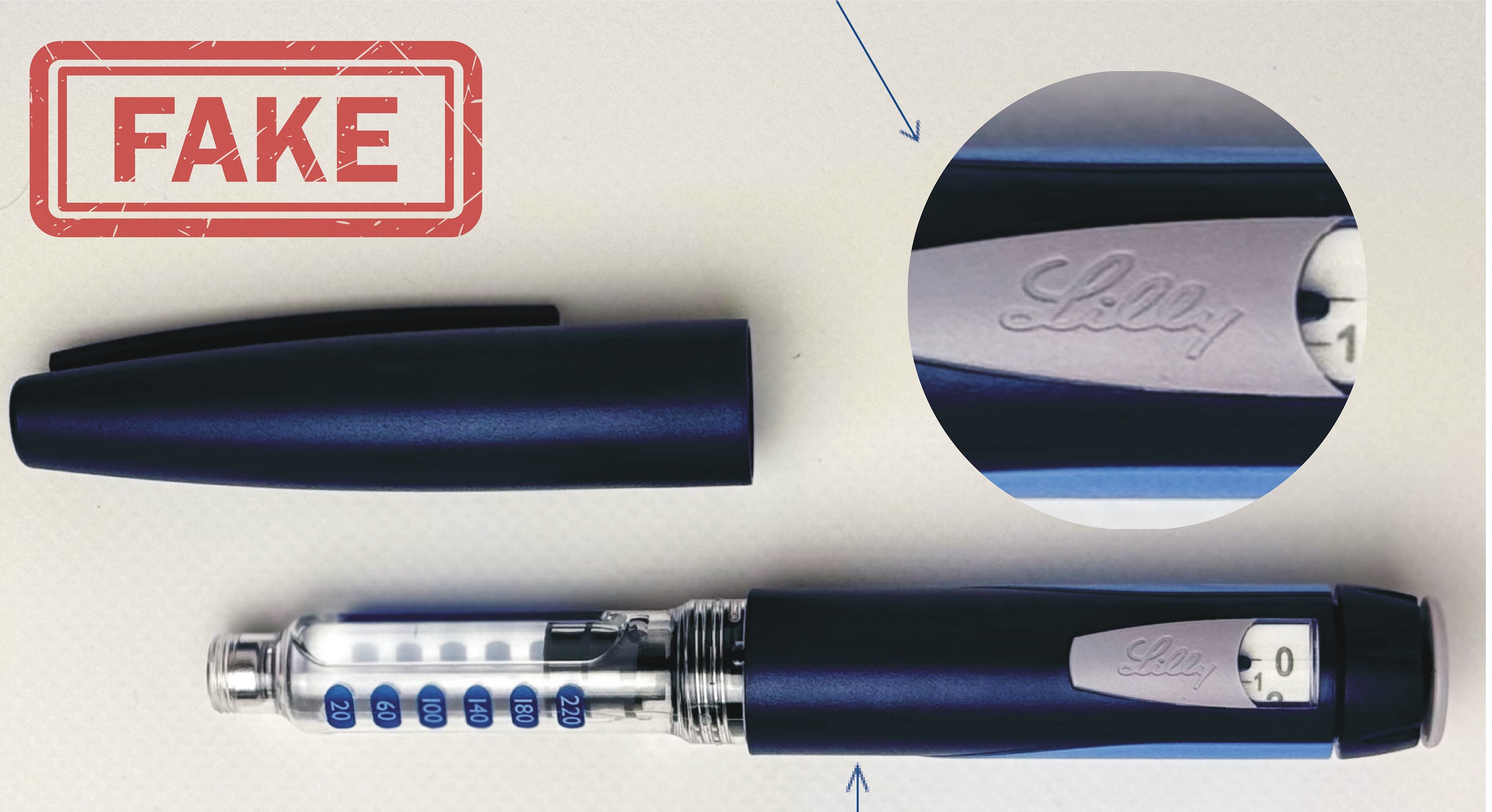
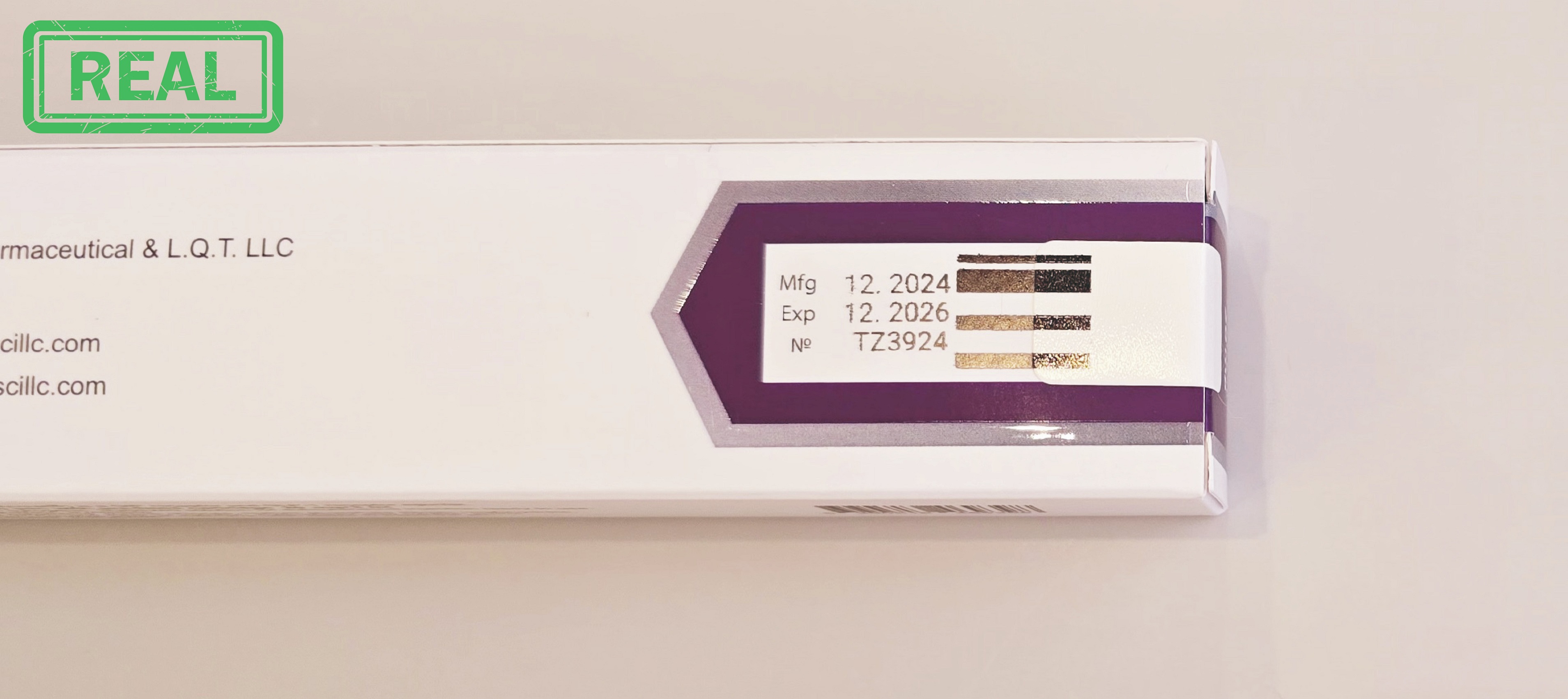
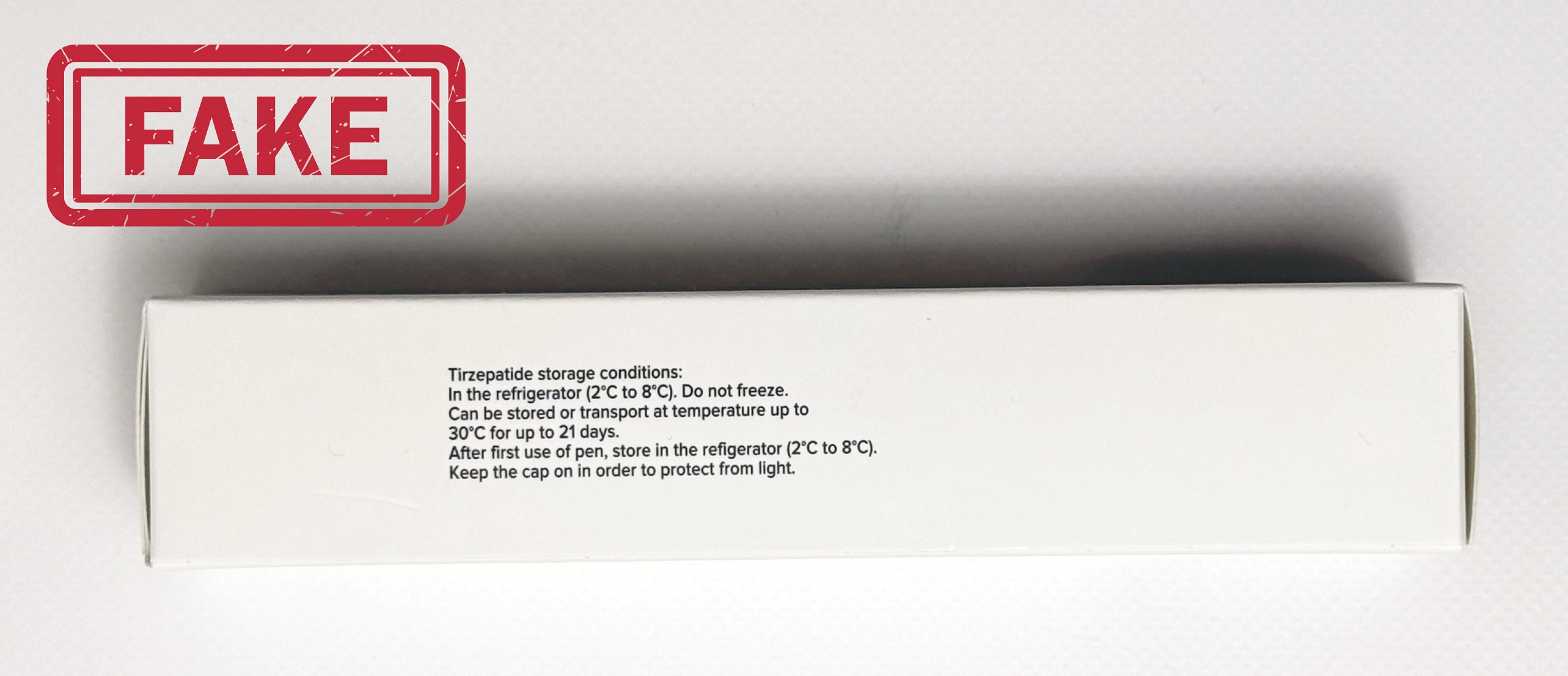
 Fake Alert
Fake Alert

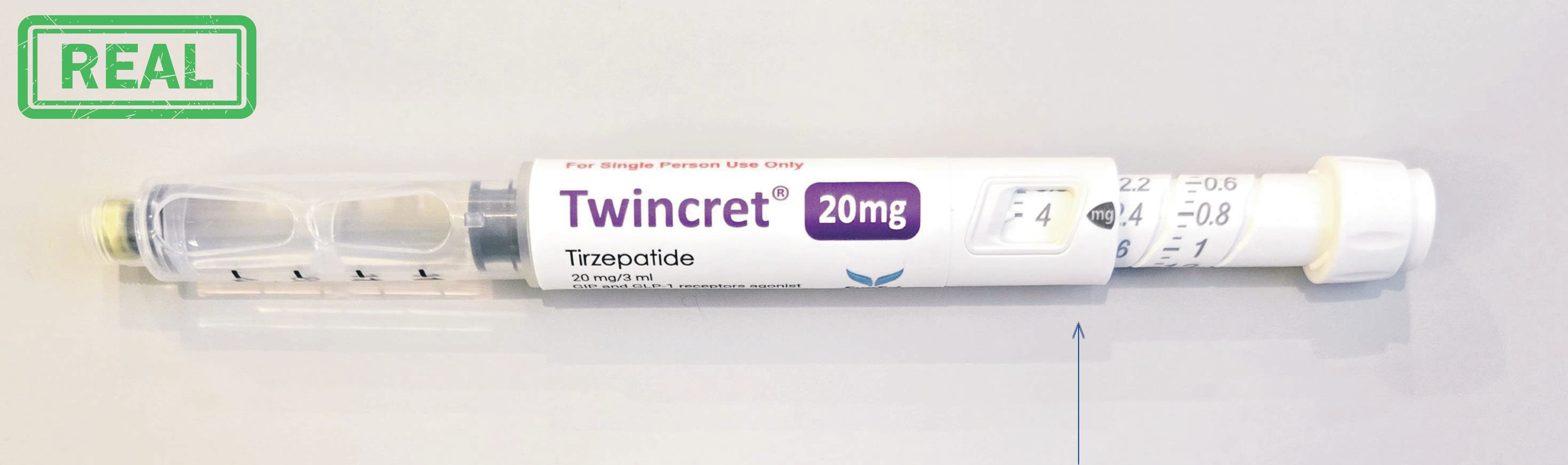
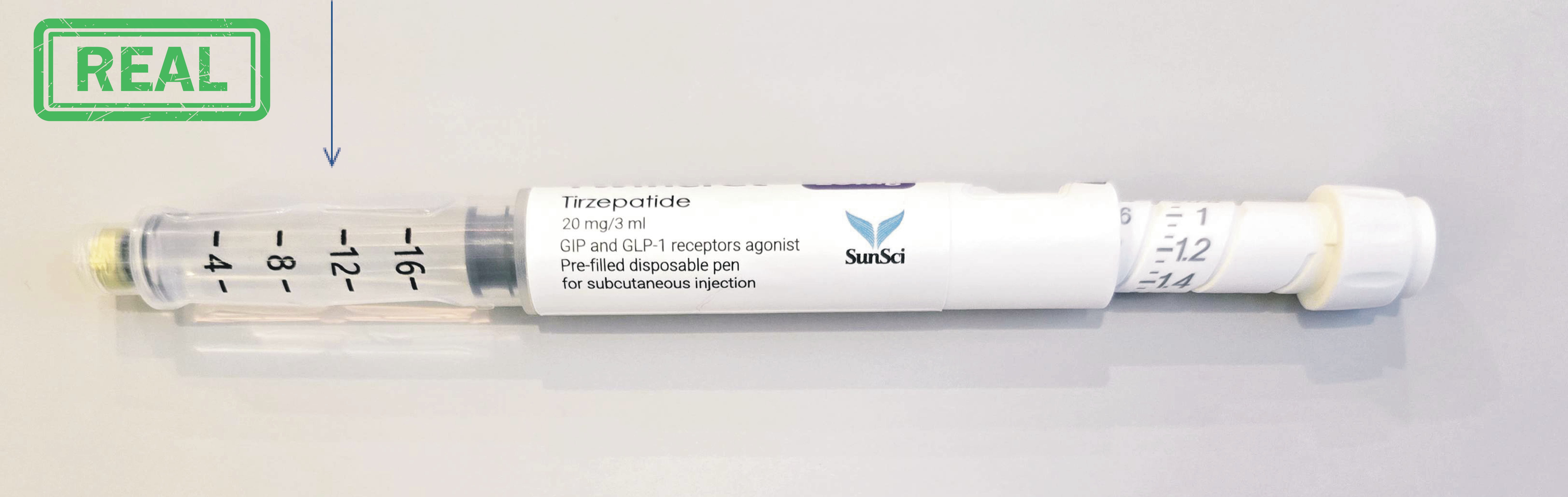

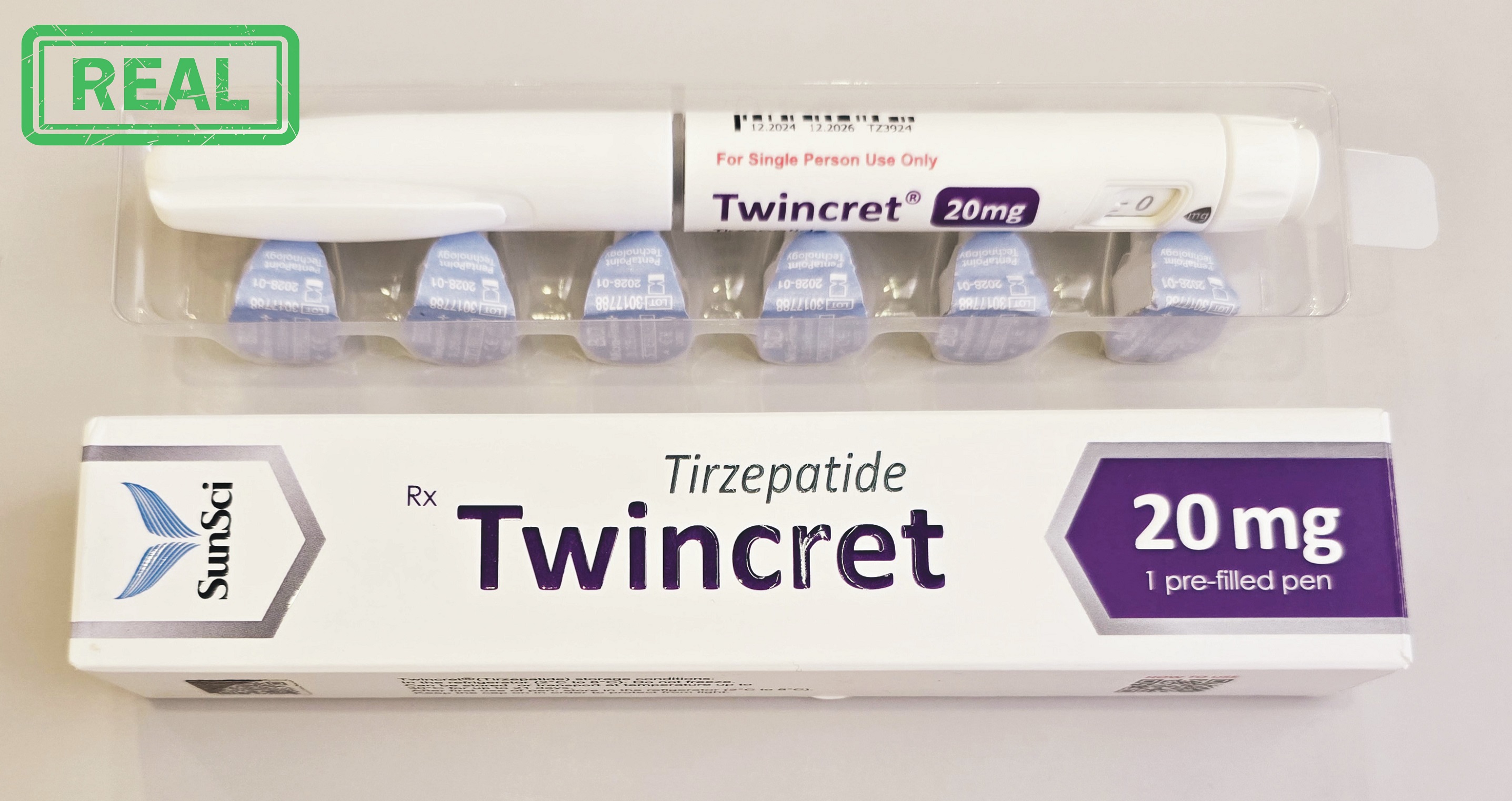















 Adiptur® Pen
Adiptur® Pen Desira® Pen
Desira® Pen Somaliq® Pen
Somaliq® Pen Tanoliq® Pen
Tanoliq® Pen Twincret® Pen
Twincret® Pen Vigelan® Pen
Vigelan® Pen Satifam® Pen
Satifam® Pen CompleNAD® Pen
CompleNAD® Pen Mitonic® Pen
Mitonic® Pen






















